Journal of Entrepreneurial Health Sciences and Innovation (JEHSI)™
Expansion and Protection of Urban Park-Biospheres: A Pressing Community Wildlife Protection Initiative in Response to Climate Change
Dr Gabriel Pulido-Cejudo1,2*
Peter Humphries2
Mary Stier-Meyer
Dr Bratati Kar1,2
Dr Khadija El Abdaimi1,2
1International Centre for Advancement of Health Regional Innovation and Science, ICAHRIS Research
*corresponding author, gabriel.pulido-cejudo@icahris.org
2Canadian Federation of Breast Diseases, CFBD
Abstract
Primarily driven by immigration, between July 1, 2022, and July 1, 2023, the population of Canada increased at a rate of approximately 3%, reaching over 40 million inhabitants. Although this increase in population within Canada has addressed the immediate and future demands for a capable and younger workforce as well as its commitment to provide refugees an opportunity to thrive and prosper, it has equally pressed upon the rapid expansion of urban and suburban areas accelerating the displacement of wildlife, loss of vital green spaces, and in some cases, the loss of otherwise irreplaceable agricultural corridors and of surrounding indigenous species of plants and animals.
This trend is not unique to Canada causing similar negative effects across North and South American countries. Relentless urban land expansion together with limited or poor environmental assessments and protection of vital indigenous habitats have been the main drivers of permanent loss of vital habitats affecting an estimated 40% of species living in native green spaces. This in turn, has increased the rate of deforestation of urban and suburban areas to service local communities contributing to and exacerbating the effects of climate change.
We present a set of concrete actions to halt and reverse this trend. These include, among other, the creation and protection of 40 - 50 km² Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) within major cities across Canada together with the permanent protection and heritage designation of all wetlands and farming corridors. The immediate need to halt trapping and killing indigenous wildlife in proximity with suburban communities is also underlined. Green under-passing corridors in areas affected by the construction of interconnecting highways are also addressed.
Keywords: Indigenous-Wildlife-Urban park biospheres (IWUPBs), deforestation, climate change, bio-diversity, indigenous species, wildlife, extinction, Pangaea Ultima/Proxima, soil micro-biome, agroecosystems, inbreeding depression, Monarch butterfly rearing
Article
Radiometric data collected from rocks on Earth, moon and meteorites, the latter being less altered by rock cycles as those found on Earth, suggests that our planet was formed approximately 4.5 to 5 billion years ago whereby the chemical composition, physical and dynamic events on Earth were significantly different from those we know today[1,2]. The most conspicuous was the reducing nature of its atmosphere primarily composed of carbon dioxide (CO2), methane (CH4) and water (H2O) vapours. As part of the early physicochemical dynamics of its early reducing atmospheric composition, water vapour molecules are believed to have been dissociated by solar energy into oxygen and hydrogen[3]. The reaction of free O2 with CH4 was swift, sequestering it into the crust of the Earth with rather minute amounts of O2 being left in its reducing early atmosphere.
The emergence of cyanobacteria almost 2.7 billion years ago would change these dynamics. Capable of carrying out anoxygenic photosynthesis under reducing anerobic atmospheric conditions leading to the production of oxygen as a byproduct of this metabolic pathway, cyanobacteria are believed to have accelerated the transition between a reducing early atmospheric condition to and oxidising atmosphere of Earth. To date the most abundant atmospheric gases are nitrogen (N2) and oxygen (O2).
It has been proposed that the explosive growth of cyanobacteria during the span of 200 - 300 years, led to the production of high levels of O2 being released into vast regions of ocean waters[3,4]. Not being able to be sequestered by ocean water minerals, it is believed that free O2 was gradually released into the atmosphere during the Great Oxygenation Event[3, 4].
To date, the increase in global human population and disruptive anthropogenic activities such as gas and oil production, mining, extensive farming, food production, industrial manufacturing, transportation, urbanisation, production and use of chemicals, forestry and deforestation, amongst several other actions, have significantly contributed to slowly restoring the reducing nature of the early atmosphere on our planet.
During the years to come, these relentless human disruptive activities risk to accelerate the current release of CO2 and CH4 concurrently taking place through volcanic activity, melting of Icelandic glaciers and, among other, the facultative aerobic continuous growth of terrestrial and aquatic cyanobacteria[4-6].
From the evolutionary perspective of our planet alone, the current trend signals the eventual reversal of its current oxidising atmosphere rich in N2 and O2 to that where the predominant composition would be once more CO2 and CH4, conditions in which, all current aerobic living species, including humans, will not survive.
Across the Cenozoic, approximately 66 million years ago to this date, mammals have successfully adapted to extreme cooling and warming temperature fluctuations dominating land and marine habitats of all continents. This dominant trait of mammals towards resilience to extreme climate fluctuations most likely will be insufficient for survival under upcoming geological transformations.
Based upon the plate tectonic theory, it has been predicted that the merging of current continents back into the next supercontinent, the Pangea Ultima also known as Pangea Proxima, could take place in the next 200 - 300 million years[7], leading to extreme and sustained temperature fluctuations making this super-continent uninhabitable for most forms of life, particularly mammals[8,9].
Although this ultimate state of ongoing geological evolutionary process of our continents is still fairly distant, anthropogenic emissions of reducing gases causing an increase in global warming through their greenhouse effect had not been detected for millions of years prior to the intense industrial activity and urbanisation taking place in the last one hundred years[9,10].
The current trend has brought us closer to reaching a global increase in temperature of 1.5 degrees Celsius above that registered in the pre-industrial era. This increment has been set and recognised by the international scientific community as that causing irreversible effects on climate change within some of the most vulnerable habitats and communities of our planet[8-11]. However, this does not imply that global increments in temperature are not cyclical depending on overall weather patterns or that critical changes have not already taken place.
Commencing in March 2023, hundreds of forest fires burned across Canada continuing throughout the entire summer. By the beginning of November 2023, almost 6,640 fires had burned approximately 185,000 km² (~46 million acres) of forests equivalent to 5% of all green forests in Canada[12,13]. It is estimated that these fires generated nearly 600 million metric tons of CO2[14].
In perspective, as weather patterns change and dryer conditions set in due to, among other factors, a continuous increase in temperature leading to a greater number of forest fires and concurrent natural disasters, the release of total greenhouse emissions will continue to attain higher levels faster than expected hence accelerating the pace of global warming.
In 2023, the 600 million metric tons of CO2 released by forest fires alone, was equivalent to almost 90% of the total greenhouse gas emissions from all sources in 2021 in Canada[14]. Concurrent with major impacts of climate change on vulnerable ecosystems and communities, deforestation, and degradation of critical habitats due to extensive and poorly planned urbanisation and related human activities have put at risk the survival of more than one million plant and animal species.
This in turn has triggered an increase in the onset of zoonotic and infectious diseases transferred from humans to animals through zooanthroponosis or reverse zoonosis[15,16]. We present a set of tangible measures and examples of concrete actions required to assist in mitigating the erosive effects of ongoing natural disasters exacerbated by climate change, relentless urban sprawling, poor urban planning and environmental assessments.
Importance of Wildlife and Resiliency Across Urban and Suburban Areas
During the course of several centuries, most civilisations have erected urban cities leading to the extensive abrogation of indigenous animal and plant species. After the radical clearing of most of the indigenous wildlife, the remaining species are confined to fragmented and insufficient green spaces capable of sustaining smaller plant and animal species forced to survive in sub-optimal mixed environments mainly composed of buildings, roads and interconnecting highways.
These human-driven mixed environments are mostly composed of non-indigenous species, often leading to the fostering of foreign invasive species further hampering the ability of indigenous wildlife to survive. This is compounded by the introduction of human pollution encompassing the use of poisonous chemicals, pesticides both chemical and biological, as well as the sustained accidental and otherwise intentional killing of the surviving indigenous plant and animal species.
To date, species affected includes vital pollinating insects, birds, amphibians and reptiles such as indigenous snakes, lizards, turtles and smaller mammals such as raccoons, badgers, skunks, rabbits, squirrels and chipmunks, to cite a few.
After being forced to occupy the remaining green spaces in suburban areas shared with agricultural corridors, the populations of larger mammals such as foxes, coyotes, wolves, deer and bears are under the constant threat of being intentionally trapped and killed, being considered dangerous nuisances by the inhabitants of the urban and suburban communities that were erected in their original habitats.
Not only this has eroded the bio-diversity of wildlife surrounding urban and suburban spaces, but it has equally rendered them less resilient to current natural disasters caused by intense flooding, hurricanes and forest fires as a direct result of climate change.
Poor urban planning and intense deforestation has led to the creation of wind corridors in areas otherwise protected by indigenous trees and vegetation. In the pursuit of maximising profit and the use of all land available, houses and buildings have been erected on irreplaceable wetlands and ancestral agricultural corridors. As a result, not only these structures are constantly being flooded, but the loss of wetlands has equally led to the loss of unique wildlife and soil micro-biome. Closely linked to indigenous wildlife, soil structure and soil micro-biome play vital roles in regulating aggregation, pore connectivity, water flow and overall soil fertility[17].
To date, sustained displacement of indigenous wildlife resulting from relentless urbanisation and poor planning has already affected soil fertility and natural water flow globally through pollution, waste of natural resources and degradation of indigenous soil micro-biome[17-18].
As the population, not only in Canada but globally, will continue to grow, it has been estimated that, merely seven years from now, urban expansion could cause the demise of close to 290,000 km² of natural habitats by 2030[19].
Based upon the assumption that the global human population will grow by 2.5 billion people by 2050, using as reference 30,393 land vertebrate species, it has been predicted that urban land expansion could affect up to 39% of these species due to habitat loss[20]. Current practices of urbanisation are clearly not sustainable.
In an effort to rapidly cater to the demands of those benefiting from financial gains as a result of urban growth as well as to the demands of a growing population with the genuine need to gain access to affordable housing, policy makers and decision makers have either disregarded or abolished science-based expert assessments aimed at securing the protection of critical habitats and indigenous wildlife. Although these deficiencies will be addressed in the Sections below, a set of examples of the resiliency of wildlife across current urban and suburban areas is provided.
Wildlife Resiliency Across Urban and Suburban Areas
Despite having reached an unprecedented stage of geopolitical instability and consequential eroding events deeply affecting wildlife, global organisations, including ICAHRIS Research, continue to make tangible contributions aimed at curbing the loss of close to 290,000 km² of natural habitats by 2030 due to relentless urban growth[19].
Concurrent to these efforts, some indigenous plant and animal species including arthropods, birds, reptiles, amphibians and an array of marine and terrestrial mammals have been forced to adapt and occupy urban and suburban habitats to survive.
This level of adaptive plasticity was distinctly witnessed during global lock-downs undertaken throughout the recent COVID-19 / SARS CoV-2 pandemic outbreak[15,16,20,21].
Sightings of wildlife roaming on the streets of urban and suburban areas in various countries, including Canada, encompassed species not seen before in these communities. Some of the animal species found in these areas are summarised in Table 1.
| Common name | Scientific name | Country |
| Black bear | Ursus americanus | Canada, United States |
| White-tailed deer | Odocoileus virginianus | Canada, United States |
| Red and black fox | Vulpes vulpes | Canada |
| Kashmiri goat | Capra aegagrus hircus | Wales |
| Jaguar | Panthera onca | Mexico |
| Lynx | Lynx canadensis | Canada |
| Moose | Alces alces | Canada |
| Sea lion | Otariinae | Argentina |
| Black wolf | Canis lupus lycaon | Canada, United States, United Kingdom |
Table 1 Sightings of wildlife species roaming the streets of urban and suburban communities during the 2020 and 2021 COVID-19 lock-downs
Sightings of wildlife across various communities globally were particularly frequent during 2020-2021 COVID-9 lock-downs. These sightings not only reflect the presence of wildlife bio-diversity within surrounding undisturbed habitats but the adaptive plasticity of its inhabitants to re-occupied their indigenous environments prior to urbanisation.
In addition to the sightings of wildlife species outlined on Table 1, Rogers, Yong and Holden, a group of scientists from the University of Queensland, Australia, and North-West University, South Africa, reported the presence of approximately 1,168 discrete plant and animal species documented during consistent daily observations performed within a single home, located in a medium-density urban housing area in the city of Brisbane, Australia[21]. A breakdown of the species found in this study is depicted in Table 2.
| Class / Order | No. of Organisms |
| Lepidoptera | 437 |
| Diptera | 110 |
| Coleoptera | 98 |
| Hymenoptera | 87 |
| Hemiptera | 79 |
| Insecta (Other) | 65 |
| Arachnida | 63 |
| Arthropod (Other) | 16 |
| Annelida | 2 |
| Mollusca | 5 |
| Amphibian | 2 |
| Reptilia | 8 |
| Aves | 56 |
| Mammalia | 11 |
| Animal (Other) | 2 |
| Fungi | 24 |
| Plantae | 103 |
Table 2 Distribution of plant and animal species determined through a systematic bio-diversity survey
Decreased human activity during 2020-2021 global COVID-19 lock-downs, provided an opportunity to unveil wildlife bio-diversity within medium-density urban housing (21). As expected, the greatest scores of species detected belong to the class Insecta and to the kingdom Plantae (green) followed by those within the Aves class (mid-dark orange). Interestingly, a good number of mammals were also systematically detected (light orange) reflecting resiliency towards highly invasive anthropogenic activities and urbanisation.
Equally performed during the 2020-2021 global COVID-19 lock-downs, this formal bio-diversity survey underscores the need to structure systematic bio-diversity assessments and programmes aimed at protecting animal and plant species found within urban-suburban spaces, surrounding green-belts and agricultural corridors.
A more comprehensive understanding of ecological communities in smaller spatial urban areas beyond public health lock-downs has proven to be essential to protect ancestral ecosystems nearby urban and suburban green spaces.
These studies have assisted in the discovery of one of the most ancient single-root-system forests ascribed to a very early rhizomorphic lycopsid, from a palaeosoil obtained in the Catskill green protected area near Cairo, NY, USA[22]. Apart from the invaluable biological data obtained from formal biodiversity surveys performed in smaller spatial green spaces to better understand and protect the dynamics of indigenous plant and animal species, under certain circumstances, they equally provide information concerning more permanent changes to terrestrial ecology due to earth's geochemical cycles, atmospheric CH4, CO2 and their impact on climate change.
These factors directly impinged into the resiliency of wildlife particularly within smaller spatial green spaces in urban and suburban communities. As poorly planned urbanisation continues to collapse vital ecosystems essential for the survival and growth of indigenous species, some urban structures have become transitional habitats for these species.
There are approximately 328 known species of hummingbirds out of which only 29 species have the ability to undertake long migrations[23,24]. Only 13 out of the 29 long migratory hummingbird species migrate between Canada, United States and Mexico[23,24]. In some places, such as in New Mexico, extensive loss of wildlife habitats has forced migratory hummingbirds to nest and reproduce in urban and suburban small special green spaces.
Despite requiring very specific environments, some hummingbirds have developed adaptive plasticity traits enabling them to successfully nest and reproduce right within urban housing structures (Figure 1A-1D).
Hummingbirds require an abundance of insects and nectars from surrounding flowering plants and trees to successfully provide for the intense feeding and nurturing of their offspring, each requiring to be fed at least two to three times a day.
Their nesting structures are also complex requiring an assortment of biological materials. Some of these materials include the bark of trees and shrubs from the Rosaceae family, moss and lichen, dry twigs and rootlets for their external supporting structure.
As shown in Figure 1 (1A-1D), hummingbird nests are roughly semi-spherical with an external diameter and height of approximately 8 cm, an internal diameter of roughly 4 cm and a depth of approximately 3 cm.
Inside, the nests are meticulously built with small white flowers, small feathers, downy fibres from a variety of plants including those from the Bromeliaceae family, spider web silk and animal fur[23,24,25].

Figure 1A 11 - 12 Days Old

Figure 1B 16 - 18 Days Old

Figure 1C 16 - 18 Days Old

Figure 1D 22 - 26 Days Old
Figure 1 (A-D) Adaptive Plasticity of Hummingbirds in Urban and Suburban Spaces
Due to the loss of wildlife habitats, some hummingbirds in New Mexico have developed the ability to nest in urban and suburban housing structures. Hummingbird chicks can fly as early as 22 - 26 days after having hatched. Their approximate size and growth rate is shown above (Fig. 1A-1D). Hummingbird nests are unevenly semi-spherical with an external diameter and height of approximately 8 cm, an internal diameter of roughly 4 cm and a depth of about 3 cm. Photographs by Mary Stier-Meyer.
It is, therefore, vital to promote and preserve the extension of urban park-biospheres where hummingbirds can find the diversity of bio-materials used for nesting as well as to find abundant feeding resources.
Migratory and non-migratory hummingbirds are at the centre of plant and animal interdependent ecological communities not only playing the role of selective pollinators but as an integral part of the evolution and adaptability of several species and ecosystems upon which we depend[25,26,27,28].
Through the northern parts of North America, including the Northwest Territories in Canada, to the Southern parts of South America, including Tierra del Fuego in Chile and Argentina, 7,000 plant species within 404 genera and 68 families depend upon at least one amongst the 361 species of hummingbirds known to date for their pollinating needs[25,26,27,28].
Regrettably, based upon Breeding Bird Survey (BBS) data collected from 1970 to 2019 and from 2009 to 2019, the populations of several hummingbird species within the Selasphorus genus have steadily declined[25]. This steady decline has been attributed to both direct human disruption of critical habitats as well as to anthropogenic activities exacerbating climate change[25].
Apart from securing the creation and protection of 40 - 50 km² Indigenous-Wildlife-Urban Park biospheres (IWUPs) within major cities across Canada, national and continental monitoring conservation and protection programmes and agreements aimed at reducing and reversing the loss of critical habitats due to urbanisation and anthropogenic activities are urgently needed.
Continuous inaction on climate change and human-driven loss of critical habitats is not an option. Combined, climate change and human disruption of vital ecological systems have led to a steady decline of close to 60% of all bird species in North America alone[25]. Since 1970, a net loss of approximately 3 billion birds has taken place with some species facing extinction within three decades[25,29].
Apart from an irreversible loss of bio-diversity impacting several ecosystems, the decline of vertebrate pollinators like hummingbirds, amongst others, will continue to impact the reproduction of a variety of plants and trees.
A recent systematic quantitative assessment of 126 experimental reports involving animal-pollinated plants and trees, estimated an average of 63% reduction in seed or fruit production due to abrogation of birds and mammal pollinators such as hummingbirds and bats among other[30].
Apart from hummingbirds, some species from the Nymphalidae family have equally adapted to urban and suburban spaces in response to habitat loss and radical decrease in their population.
Migratory butterflies such as the Monarch butterfly are one of the most remarkable examples of resiliency and adaptive plasticity.
Primarily driven by the use of neonicotinoid insecticides in the 1990s, human disruption of vital habitats, climate change and environmental pollutants have caused a dramatic decrease in the overwintering populations particularly within Western North America[31].
From a population of millions of overwintering Monarch butterflies, the population in the southern parts of California plummeted to barely 2,000 in the summer of 2020[31]. Through remarkable adaptive plasticity and resilience, Monarch butterflies have been able to establish winter breeding sites within urban areas of California, helping them to increase their population to approximately 300,000 as estimated during the autumn of 2021 and that of 2022[31].
This singular level of adaptation seems to have also taken place in southeastern states such as Florida, where it has been possible to raise Monarch butterflies within urban housing spaces and backyards nearby indigenous untouched green belts and farmland (Figures 2A-2H).
In the particular case of Monarch butterflies (Danaus plexippus), the interfacing of wildlife indigenous ecosystems with urban and suburban areas has enabled the opportunity to reduce the impact of some natural predators through selective conservation and the ability to complete a full life cycle by means of performing non-captive free-rearing activities (Figures 2A-2H).

Figure 2A Eggs are laid primarily on the foliage of milkweed plants (Asclepias syriaca). 3 - 5 days after being laid, they hatch into larva. The larva stage lasts between 11 - 18 days.

Figure 2B Monarch butterflies larvae reach the chrysalis stage after 11 - 18 days. Chrysalis stage lasts between 8 - 14 days.

Figure 2C After 7 days of pupation within the chrysalis shell, the wings and body of a Monarch butterfly become visible.

>Figure 2D As early as 7 - 8 days of pupation, the wings of a Monarch butterfly are deployed.

Figure 2E Approximately one hour after their emergence, Monarch butterflies keep extending their wings as they have access to the remaining fluids within the chrysalis shell.

Figure 2F During the first hours after being fully released from the chrysalis shell, Monarch butterflies gather energy to commence flapping their wings.

Figure 2G Immediate access to nectar and water sources are essential for Monarch butterflies to continue drying and strengthening their wings and fly.

Figure 2H Protection against predation within suburban and urban areas has enabled the successful completion of Monarch butterflies' life cycle from eggs to healthy butterflies released back into the wild.
Figure 2 (A-H) Adaptive Plasticity of Monarch Butterflies in Urban and Suburban Spaces
Some insects from the Nymphalidae family have been able to adapt to urban and suburban spaces in response to relentless habitat loss and acute decrease in their population.
Migratory butterflies such as the Monarch butterfly are one of the most remarkable examples of resiliency and adaptive plasticity. In southeastern states, such as in Florida, it has been feasible to successfully complete the full life cycle of Monarch butterflies within urban housing spaces and backyards nearby indigenous untouched green belts and farmland. Non-captive free rearing and photographs by Mary Stier-Meyer.
Non-captive free rearing of Monarch butterflies can be effectively used for educational purposes as well as to provide an opportunity to perform tagging and scientific research. Apart from raising awareness about the pressing need to curve the potential extinction of Monarch butterflies, these activities can be used to better understand and pinpoint the exact ecological degradation and loss of vital habitats resulting from erosive human activities. By contrast, captive rearing of Monarch butterflies leading to direct and indirect commercial activities does not provide a valuable path to increase Monarch butterfly populations and hence this activity should be discouraged.
The direct dependence of Monarch butterflies to milkweed plants (Asclepias syriaca) during their entire life cycle has changed the perception of this plant by the agricultural sector. During the last decade, farmers in Canada and the United States have diversified their commercial crops by introducing milkweed plantations[32,33].
Not only they attract pollinators such as the Monarch butterflies, their seeds and floss have proven to be a valuable commercial asset particularly under continuous changes in weather patterns impinged by climate change. Milkweed plantations have proven to be a unique diversification agricultural asset making farmers less dependent on traditional crop plantations and on their susceptibility to climate change. The perennial nature of milkweed is also an attractive trait in relation to agricultural dynamics and economics[33].
In addition to the direct benefits to the ever-steep declining population of Monarch butterflies, milkweed plantations have rendered a more ethical and hypoallergenic insulation material for winter garments in lieu of goose down. Furthermore, floss fibres have proven to provide a more suitable absorbent material to control and remove oil spills[34]. Due to its soft, buoyant and lustrous properties, milkweed floss has also been used in a variety of floating devices, bedding and clothing materials.
Although the examples provided illustrate the direct and indirect benefits resulting from concrete efforts aimed at wildlife preservation and that of indigenous green spaces, wetlands, water bodies and oceans, the urgent need to curve the loss of global bio-diversity should not be associated only with financial gains.
For more than a decade, it has been established that large apex consumers play a pivotal interplay in the stability and sustainable evolution of global ecosystems and their geomorphology[35].
Indiscriminately hunted for their pelts during the 18th and 19th centuries, by 1929, sea otters (Enhydra lutris) on the coast of British Columbia were extirpated, reaching unprecedented low population levels in the western oceanfront of Canada and the United States[36]. From an estimated global population between 150,000 to 300,000 prior to the fur trade, by 1911 the population of sea otters around the world had declined to merely 2,000 animals or less[37].
Concerted inter-institutional and international efforts to protect sea otters (Enhydra lutris) commenced in 1911. During 1911, Canada (then represented by Great Britain), the United States, Japan and Russia signed The International Fur Seal Treaty.
In Canada, sea otters were included within the Species at Risk Act (SARA), and their status has been systematically assessed by the Committee on the Status of Endangered Wildlife in Canada (COSEWIC), since 1978. In 1978, sea otters were designated as Endangered Species without any change until 1986[36]. In 2007, this designation was adjusted to that of Species of Special Concern[36].
More than a century after the signing of the International Fur Seal Treaty, the global populations of sea otters have started to bounce back. Although precise assessments need to be carefully validated, more rigorous conservation and management efforts have led to an increase in the global sea otter population from approximately 2,000 in 1911 to approximately 150,000 in 2019[38].
Unrelated to their sheer commercial value that, during the 18th and 19th centuries put sea otters at risk of extinction, their slow recovery has already proven their central role in the stability of global ecosystems and their geomorphology.
Sea otters are a keystone predator within coastal ecosystems. After a lengthy recovery of sea otters, re-colonisation of sea otters within the Elkhorn Slough estuary of the Monterey Bay in California has resulted in re-establishing geomorphic stability of coastal wetlands hence preventing further degradation and erosion of its coastline[39].
Apart from rising sea levels caused by climate change, loss of coastline is also driven by the vast consumption of wetland plant biomass by, amongst others, burrowing crabs (Uca sp.). As keystone predators, sea otters have kept in check the population of burrowing crabs, indirectly enhancing the permanency of coastal wetlands and of other ecosystems. These dynamics have been achieved without affecting the overall survival rates of burrowing crabs as well as of their role in the stability of coastal wetlands[39].
This elegant and clear example of the tangible benefits of restoring and protecting indigenous wildlife dynamics, habitats and ecosystems emphasises the urgency of preventing extirpation and extinction of species caused by erosive anthropogenic activities in the first place.
Even though the extinction of sea otters (Enhydra lutris) was halted through more than a century of inter-institutional and international conservation and protection endeavours, the severe reduction in their numbers led to a pronounced population bottleneck[40]. As a result, loss of genetic diversity and potential inbreeding depression has been documented[40] (see Figure 3).
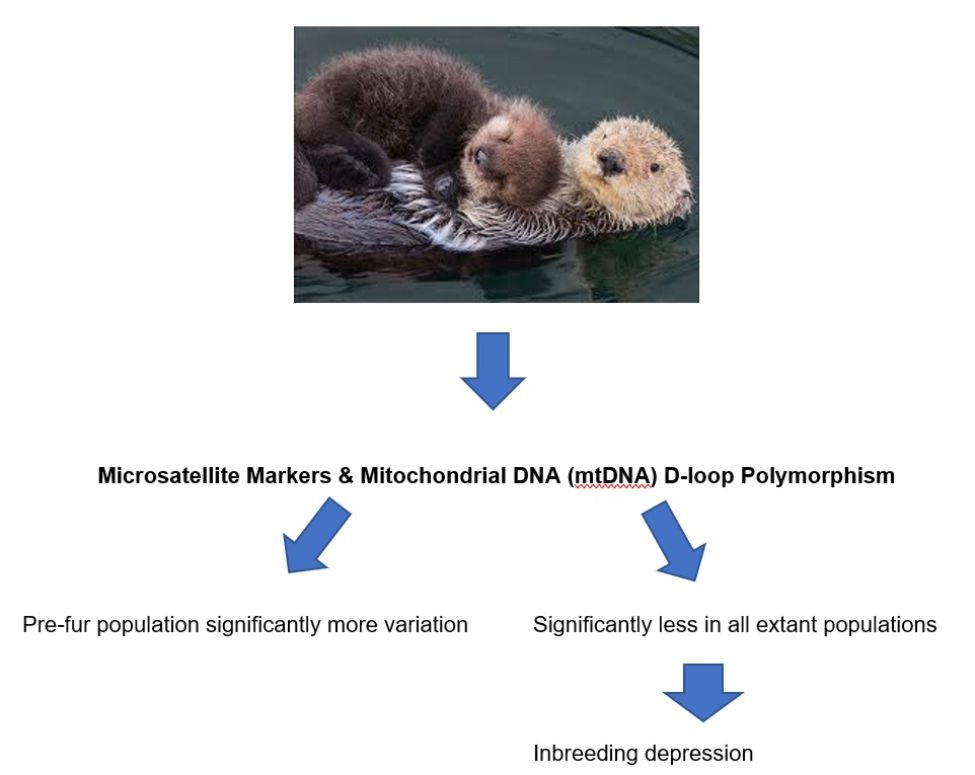
Figure 3 Potential inbreeding depression of Enhydra lutris induced by loss of genetic diversity impinged by fur trade during the 18th and 19th centuries.
Variation at four Microsatellite loci (Mvi 57, Mvi 87, Mvis 72 and Lut 453) as well as within mitochondrial DNA (mtDNA) D-loop region collected from 34 pre-fur trade DNA samples and from 5 extant otter populations from Amchitka Island, Prince William Sound, Southeast Alaska, Washington and California revealed loss of genetic diversity amongst surviving sea otter populations potentially causing inbreeding depression[40].
The recent discovery of the key role of Enhydra lutris in the permanency of coastal wetlands and of interrelated ecosystems underlines the importance of articulating durable protecting measures to avoid bottle necks and the potential onset of inbreeding depression reducing the biological fitness of vital species affected by erosive anthropogenic activities and irreversible degradation of vital ecosystems.
Creation and Protection of 40 - 50 km² Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) within major cities across Canada
Primarily driven by the sustained growth of the global human population accompanied by disruptive anthropogenic activities such as gas and oil production, mining, extensive farming, food production, industrial manufacturing, transportation, urbanisation, the production and use of chemicals, forestry and deforestation, to cite a few, we are facing an estimated loss of nearly 290,000 km² of natural habitats by 2030 due to relentless urban growth[19].
Under the supposition that global human population could increase by 2.5 billion people by 2050, it has been anticipated that urban land expansion could affect up to 39% of land vertebrate species alone induced by irreversible habitat loss[20]. Existing practices of urbanisation are outdated and visibly unsustainable.
The compounding effects of human population growth and of climate change causing more frequent environmental catastrophic events continue to negatively impact wildlife in Canada and in most countries around the world. From the estimated 8 million plant and animal species living on our planet Earth, up to 1 million are now facing extinction within upcoming decades[41].
The long-term survival of more than half a million terrestrial and marine species depends upon the immediate and sustained restoration of land and marine natural habitats. Inaction could lead to the extinction of more than 40% of amphibians, 33% of corals and fish and 33% of marine mammals, amongst others[41].
Since the 1500s, disruptive human actions have caused the extinction of close to 700 vertebrate species[41]. More precisely, and from a broader time line, since the establishment of modern humans in the Late Pleistocene[42], it has been recently estimated that humans have directly and indirectly caused the extinction of at least 1,300 to 1,500 bird species, at a rate 60 to 95 times the background extinction rate[43]. This translates into humans being the trigger of extinction of more than one in nine bird species, leading to acute and possibly irretrievable ecological and evolutionary outcomes[43].
According to a recent process-explicit macro-ecological model of climate-human-woolly mammoth interactions, using pattern-oriented modelling methods, it has been proposed that population decline, and the eventual extinction of the woolly mammoth (Mammuthus primigenius) was equally accelerated by humans combined with loss of habitat and climate change[44,45].
Inbreeding depression, primarily caused by human-induced extremely reduced population size such as that experienced by Danaus Plexippus and Enhydra lutris (Figures 2 and 3) must be halted. Inbreeding depression could lead to reduced genetic diversity, and the survival capacity of affected species could be reduced, accelerating wildlife extinction in the upcoming decades[40].
Canada, like all other global leading economies, is at a critical juncture. It has been estimated that in 2023, Canada lost approximately 5% of its forests due to persistent wildfires and related catastrophic events[13]. There is mounting evidence that sustained disruptive anthropogenic activities have pointedly contributed to gradually bringing back the reducing nature of the early atmosphere of our planet and, hence, the induction of climate change and the onset of wildfires and other intense and more frequent natural disasters, globally.
Apart from the severe loss of infrastructure and displacement of affected communities during the 2023 wildfires across Canada, several plant and animal species were destroyed, and, in some cases, wild animals were forced to seek refuge in urban and suburban communities. Due to the lack of adequate training and capacity to attend promptly and efficiently to the needs of wild animals seeking refuge, these animals were killed rather than providing them with adequate treatment, rehabilitation and release back into their original habitats.
As cited earlier, relentless human-driven habitat loss and over-exploitation, combined with climate change and severe environmental pollution, has put wildlife on the course of mass extinction whereby migratory animal species have been disproportionately impacted[46,47].
Global, immediate and concerted efforts are required to reverse this trend. To this effect, it has been recently reported that protected areas play a pivotal role in buffering the combined deleterious influences of climate change and habitat loss on wildlife[48].
Based upon known population dynamics and the minimal space range to ensure survivorship of several migratory and non-migratory wildlife species[20,24,30,32,39,41], we propose the creation and protection of Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) within major cities across Canada. These Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) must encompass the following characteristics, protective guidelines and supporting programmes.
Indigenous-Wildlife-Urban Park Biospheres (IWUPBs)
Characteristics
1. Regardless of their population size, all urban and suburban cities should allocate 40 - 50 km² of Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) buffering green spaces across all municipalities located between otherwise highly dense populations.
2. Discontinuous IWUPS buffering green spaces should encompass safe green underpasses to prevent accidental killing of wildlife by traffic and / or contact with domesticated animals.
3. In agricultural corridors that interface with IWUPS buffering green spaces, the use of organic farming practices should be encouraged, avoiding the use of chemicals such as pyrethrin and growth regulators including chlormequat chloride. Co-cultures of crops of commercial interests and those facilitating the recovery of endangered species such as the Monarch butterflies (Danaus plexippus), such as milkweed, should also be preferred in these agricultural spaces.
Protective Guidelines
1. Indigenous-Wildlife-Urban Park Biospheres (IWUPBs), agricultural corridors and wetlands, directly or indirectly connected or interfacing with IWUPS buffering green spaces, should be designated as heritage conservation lands, preventing any urban development or infrastructure within these buffering green spaces.
2. In the event of the inevitable contact with indigenous animals such as bears, wolves, cayotes foxes, raccoons and skunks, amongst others, potentially roaming suburban and urban areas in search of food, water and / or shelter against natural catastrophic events such as wildfires, the current practice of trapping and killing these animals must be halted.
3. Green underpasses, natural and / or man-made barriers should be built in areas disturbed by the construction of interconnecting highways or major rural roads.
4. Houses and servicing infrastructure must encompass buffering green spaces and materials suitable to enable the continuous growth of indigenous wildlife plants and animals in accordance with the findings of urban and suburban systematic bio-diversity surveys. The use of poisonous substances and herbicides and insecticides of any kind, particularly those affecting Monarch butterflies, such as those containing pyrethrin, should be discontinued.
Supporting Programmes
1. Comprehensive bio-diversity censuses of urban properties in proximity with Indigenous-Wildlife-Urban Park Biospheres (IWUPBs), agricultural corridors and wetlands should be implemented, supported and coordinated by all appropriate federal departments including, amongst others, the ministries of Environment and Climate Change, Agriculture and Agrifood, Science and Innovation, Fisheries and Oceans, Energy and Natural Resources and Global Affairs.
These systematic biodiversity assessments and programmes must be aimed at protecting animal and plant species found within urban-suburban spaces, surrounding greenbelts and agricultural corridors. Data obtained from formal biodiversity surveys performed in smaller spatial green spaces not only aid in better understanding and protecting the dynamics of indigenous plant and animal species, but they could also provide evidence concerning more permanent changes to terrestrial ecology due to Earth’s geochemical cycles, atmospheric CH4, CO2 and their impact on climate change.
2. Bioremediation and reclamation of disturbed and / or contaminated abandoned lands in communities affected by mining, forestry and oil extraction should be accelerated and transformed into Indigenous-Wildlife-Urban Park Biospheres (IWUPBs).
3. In collaboration with local schools and educational institutions, organise quarterly activities aimed at identifying, preserving and cultivating plant species prevalent within Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) and surrounding green spaces, raising awareness of their benefits in sustaining local wildlife conservation and biodiversity.
4. Concomitant with the current rush to build new houses and servicing infrastructure, ensure that no active or previously designated agricultural and farming areas are re-purposed for housing projects but rather for the creation of Indigenous-Wildlife-Urban Park Biospheres (IWUPBs).
5. Any new urban and suburban construction project, including new housing and the expansion of current roads and highways, should include the protection of indigenous wildlife and the simultaneous bioremediation and reclamation of disturbed and / or contaminated abandoned lands and their conversion into Indigenous-Wildlife-Urban Park Biospheres (IWUPBs) elsewhere and regardless of their proximity to new construction projects.
We are currently at the crossroads of human accelerated climate change, ubiquitous pollution and unsettling loss of wildlife bio-diversity, whereby an estimated 13% of all plant and animal species, equivalent to close to 1 million living organisms, are facing extinction in the upcoming decades[41].
More than ever before, human ingenuity and ability to halt and to reverse severely disruptive anthropogenic actions are essential to ensure our own survival. Planet Earth is not for us to exploit at our leisure but to protect, share and functionally acknowledge our intertwined coexistence with all living species and their resiliency to survive.
The sightings of wildlife within numerous urban and suburban communities globally during 2020-2021 COVID-19 lock-downs reflect the resilient presence of wildlife bio-diversity within nearby remaining undisturbed habitats and their ability to reclaim their indigenous environments prior to urbanisation. The successful reclaim of indigenous habitat environments by wildlife has been particularly remarkable within the Chernobyl Exclusion Zone (CEZ).
After the complete human evacuation from the CEZ due to the nuclear power reactor meltdown in Chernobyl in 1986, several indigenous plant and animal species rapidly repopulated indigenous habitats, showing a remarkable mitochondrial diversity most likely induced by sustained exposure to ionising radiation for close to 40 years[49]. This remarkable example of genetic resiliency and adaptation underlines the utmost importance of providing buffering biospheres within indigenous green spaces, where wildlife can resettle and prosper.
We can and we must change course for the betterment and the enduring survival of all living species on Earth, some of which pre-date the Chicxulub asteroid mass extinction of dinosaurs. These include plants like the Wollemi pine (Wollemia nobilis), Sago cycad (Cycas revoluta), Hard fern (Blechnum spicant), Horsetail restio (Elegia capensis), Soft tree fern (Dicksonia antarctica) and Magnolia trees, as well as several species of insects including beetles and cockroaches, reptiles such as alligators and crocodiles, sharks and birds.
Mankind cannot afford another legacy of anthropogenic-induced mass extinctions.
Acknowledgments
The authors would like to acknowledge the exceptional contributions and remarkable commitment of citizen entomologist Celeste Lehrer toward educating the next generations about the utmost relevance of protecting and enhancing the ability of endangered insects such as the Monarch butterflies to circumvent extinction through community programmes and creative teaching initiatives.
For reprints, please use the contact form, below, or address the corresponding author, listed above.
