Journal of Entrepreneurial Health Sciences and Innovation (JEHSI)™
Prospect of the Combined Use of Lysosomotropic Agents, Gene-Editing and
nNE Adjuvant Biogenic Clones for the Effective Prevention of
Community Transmission of SARS-CoV-2/COVID-19 and Emerging Coronavirus Infections
Dr Gabriel Pulido-Cejudo1,2*, Peter Humphries2, Dr Khadija El Abdaimi1,2, Dr Bratati Kar1,2 and Dr Abraham Pulido-Cejudo3
1International Centre for Advancement of Health Regional Innovation and Science, ICAHRIS
*corresponding author, gabriel.pulido-cejudo@icahris.org
2Canadian Federation of Breast Diseases, CFBD
3Hospital General de México
Abstract
A detailed analysis and timelines on the origins of the ongoing SARS-CoV-2/COVID-19 pandemic outbreak is provided. Based upon the common binding of the viral spike peptide of SARS-CoV, the causative agent of SARS, and that of the spike peptide of SARS-CoV-2 to their putative receptor Angiotensin Converting Enzyme 2 (ACE2), a potential early and late viral entry of SARS-CoV-2 through a Clathrin and Caveolae-independent endocytic pathway followed by a pH-dependent intracellular processing is proposed. The prospect of the combined use of lysosomotropic agents, gene-editing and the use of nNE adjuvant biogenic clones to hinder viral infection and community transmission of SARS-CoV-2/COVID-19 is described. Three pressing global strategies to reduce ongoing and emerging outbreaks associated with Severe Acute Respiratory Syndrome Related Coronavirus (SARS-CoVs) are also proposed.
Keywords: Lysosomotropic Agents, Gene-Editing, nNE Adjuvant Biogenic Clones, Prevention, Community Transmission, SARS-CoV-2, COVID-19, Coronavirus Infections, SARS, MERS
Article
SARS-CoV-2/COVID-19 Outbreak: First Broad-Spectrum Reports
Throughout the course of the last days of December, 2019, information first shared on public media described the emergence of new cases of pneumonia of unknown origin taking place in the city of Wuhan, capital of the Hubei province, located in central China, with an estimated population of 11 million inhabitants.
Some of these early broad-spectrum reports have been rightfully attributed to ophthalmologist Dr Li Wenliang, who had been working at Wuhan Central Hospital since 2014[1]. On December 30, 2019, Dr Wenliang unofficially and privately shared with some colleagues the emergence of a disease that shared some close resemblances to the severe acute respiratory syndrome (SARS) illness that first took place on December 16, 2002, in the Guangdong Province of China, near Hong Kong.
He shared his observations after apparently having seen seven patients with clinical signs similar to those detected during the SARS outbreak of 2002[1]. Based upon the sharing of this potential SARS-like outbreak that could explain the growing number of individuals suffering from pneumonia of unknown origin, Dr Li Wenliang was among the eight people detained by Wuhan Chinese authorities, a few days after having shared his medical observations, deemed, at the time, to have been rumours by the corresponding authorities[1].
In spite of having been warned about being involved in illegal activities with the potential of facing legal punishment, Dr Li Wenliang continued his clinical work at Wuhan Central Hospital. On December 31, 2019, Wuhan health authorities reported that 27 people, the majority of them vendors at the Huanan Seafood Wholesale Market, had been treated in hospital; seven patients were considered to be in serious condition, perhaps alluding to the same seven patients earlier mentioned by Dr Li Wenliang.
On the following day, January 1, 2020, Wuhan authorities apparently confirmed that, in addition to seafood, the Huanan Seafood Wholesale Market sold a variety of animals including exotic birds, bats and other wild animals, some of which were apparently destined for human consumption. On the same day, January 1, 2020, the authorities ordered the immediate closure of this market and its decontamination.
This event and related reports also triggered the attention of surrounding health authorities, including those within Hong Kong, seeking clinical pathological analysis searching for a viral origin of the escalating cases of pneumonia within the region.
On January 7, our organisation shared the findings of a group of Chinese scientists who discovered a new strain of coronavirus, signalling that this strain was most likely the causative agent of all cases of pneumonia of unknown origin detected since late December 2019. Almost a month later, on February 7, 2020, Dr Wenliang would die of the disease at the age of 33[1], prompting a closer investigation of the actual causes of his death by the National Supervisory Commission within China. It appears that, on March 20, 2020, the Chinese government formally exonerated Dr Li Wenliang, and it is believed that the ruling communist party accepted having committed an error.
Early Identification of SARS-CoV-2 coronavirus (previously 2019-nCoV) from a
Betacoronavirus 2β Lineage as the Causative Agent of Coronavirus Disease (COVID-19)
After recent access and a systematic review of current published data and official reports, it appears that, during the month of December 2019, the first incidence of pneumonia of unknown origin may have taken place as early as December 8, 2019, within the city of Wuhan, capital of Hubei province, located in central China,[2-6].
The first cluster of seven patients was admitted to the intensive care unit (ICU) of the Wuhan Jin Yin-Tan Hospital at the beginning of the outbreak between December 20 and December 29 (see Table 1). Common underlining symptoms of these patients were fever, shortness of breath (dyspnoea), palpitations, chest tightness, headache, expectoration and unresponsive treatment of pneumonia with antibiotics. The common lack of response to antibiotic treatment within this first cluster of patients ultimately led to the search of a potential viral causative agent.
Following the consent of patients, oral and anal swabs, as well as blood and bronchoalveolar lavage fluid (BALF), of a critically ill patient were collected for viral culture and RNA extraction[2]. On or about December 30, 2019, Peng Zhou and a group of scientists within his team conducted analysis that led to the isolation of a new strain of coronavirus. They found that three different bronchoalveolar lavage samples (BALFs) were positively reactive to pan-Betacoronavirus using Real-Time PCR (RT-PCR) assays[2].
Sequencing of the complete virus genome at depth, using combined Illumina-based within-sample diversity of the entire viral genome together with viral genome-length reads encompassing mutations within single virus particles obtained through Nanopore technology[7-8], have consistently shown that the new coronavirus strain fits within the Betacoronavirus 2β lineage[2-6].
Original isolates from the early cluster of patients from Wuhan showed a strong homology with bat SARSr-CoV RaTG13 (96.2%) while a weaker one with human variants of SARS-CoV, the virus responsible of Severe Acute Respiratory Syndrome (SARS)[2].
The identification and direct association of the novel coronavirus SARS-CoV-2, originally identified under the putative name of 2019-nCoV[2], to the emerging coronavirus disease COVID-19, is based upon the high level of sequence identity (99.98%) found between individual SARS-CoV-2 strains isolated from different clusters of patients residing at the epicentre of the first outbreak reports of COVID-19 within China[2,6].
Timeline Summation of Current SARS-CoV-2/COVID-19 Outbreak
Following an extensive and detailed analysis of unpublished original reports, communications and, more recently, a prolific release of scientific publications, it has become fairly certain that the first case of COVID-19 took place on December 8, 2019[3]. Figure 1 summarizes some of the key events of current SARS-CoV-2/COVID-19 outbreak.
| Patient ID | Gender | Date of Onset | Date of Admission | Symptoms | Status up to 13-Jan-2020 | Blood IgM Against Tested Pathogens* |
| ICU-01 | Male | 62 | 27-Dec-2019 | First episode with fever on 12-Dec-2019. Recovered no medical treatment required. Relapsed with wife also presenting symptoms. Re-admitted on 27-Dec-2019 with fever. | Recovered, discharged. | Negative |
| ICU-04 | Male | 32 | 29-Dec-2019 | Fever, cough, dyspnoea. | Fever, intermittent cough. | Negative |
| ICU-05 | Male | 40 | 27-Dec-2019 | Fever (38°C) expectoration, malaise, dyspnoea. | Fever, malaise, intermittent cough. | IgM Adenovirus |
| ICU-06 | Female | 49 | 27-Dec-2019 | Fever (37.9°C), palpitations. | Fever, malaise, cough. | Coronavirus (nt) |
| ICU-08 | Female | 52 | 29-Dec-2019 | Fever (38.5°C), expectoration, malaise, dyspnoea. | Recovered, discharged. | Streptococcus pneumonia (nt) |
| ICU-09 | Male | 40 | 28-Dec-2019 | Fever (38.5°C), expectoration. | Fever (38.5°C), malaise, expectoration, dizziness. | Negative |
| ICU-10 | Male | 56 | 20-Dec-2019 | Fever, dyspnoea, chest tightness. | Fever, malaise, cough, dyspnoea. | Negative |
Table 1 Early cluster of patients suffering from pneumonia of unknown origin
Data shown on Table 1 was reported as extended patient information collected at the Intensive Care Unit (ICU) of the Wuhan Jin Yin-Tan Hospital by Zhou, P et al.[2]. Patients outlined on Table 1 were tested for the presence of blood IgM against a panel of respiratory pathogens that encompassed:
| Legionella pneumophilia | Mycoplasma pneumoniae | Chlamydia pneumoniae |
| Respiratory Syncytial virus | Adenovirus | Rickettsia |
| Influenza A virus | Influenza B virus | Parainfluenza virus |
All patients, with the exception of ICU-05, who showed IgM antibodies against Adenovirus, were negative against the panel of respiratory pathogens tested. With the exception of patients of ICU-01 (who was first admitted on 12-Dec-2019) and of patient ICU-08, who were discharged on 13-Jan-2020, all other patients remained in hospital between 16 to 25 days, at least, after being admitted. Although the exact individual outcome and total number of days in hospital were not reported, the available information indicates that intensive care could have taken up to 3.5 weeks.
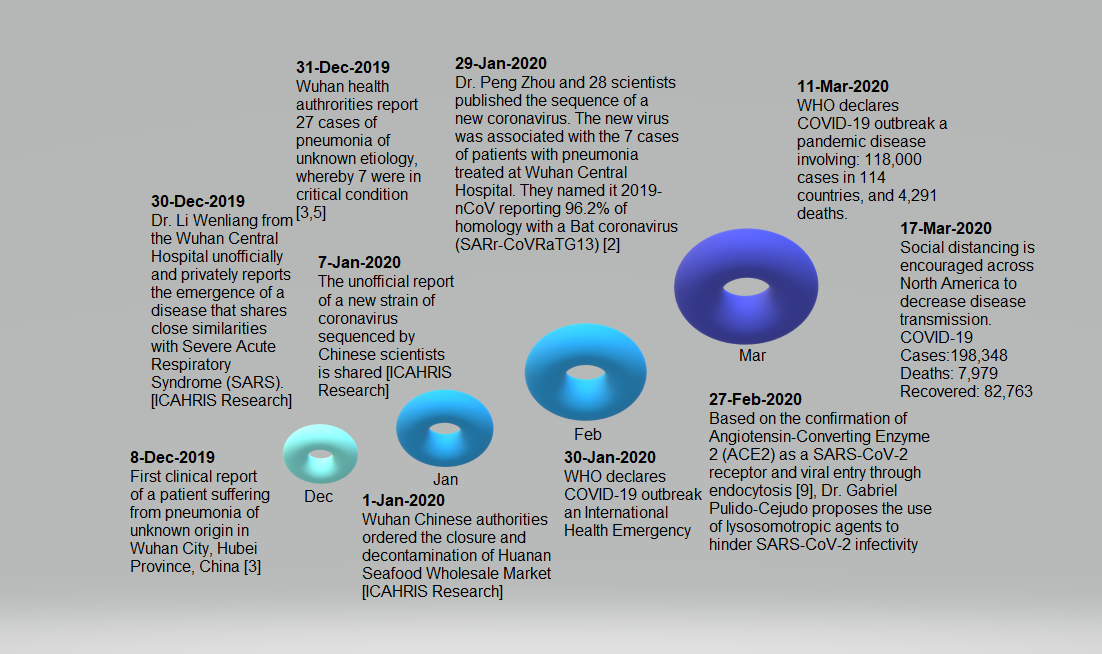
Figure 1 Critical timeline summation of SARS-CoV-2/COVID-19 pandemic outbreak
The first clinical report of a patient suffering from pneumonia of unknown origin, now identified as COVID-19, took place on December 8, 2019, in Wuhan city, Hubei China. Later reports of seven more people infected and admitted at the Intensive Care Unit (ICU) of Wuhan Central Hospital were reported by Dr Li Wenliang on December 30, 2019. Dr Li Wenliang contracted SARS-CoV-2, the causative agent of COVID-19, while working at the Wuhan Central Hospital. Dr Wenliang died on February 7, 2020, at the age of 33. Using biological samples apparently obtained from the same patients unofficially reported by Dr Wenliang, Dr Peng Zhou and 28 scientist colleagues published the first entire sequence of SARS-CoV-2, originally naming it 2019-nCoV. They found that SARS-CoV-2 is 96.2% homologous to a bat coronavirus (SARSr-CoV RaTG13), suggesting a possible transmission from bats to humans. On February 27, 2020, based upon the confirmation of Angiotensin Converting Enzyme 2 (ACE2) as a receptor of SARS-CoV-2[9], Dr Gabriel Pulido-Cejudo proposed the use of lysosomotropic agents to hinder SARS-CoV-2 infectivity and, hence, community transmission. On March 11, 2020, WHO declared COVID-19 a pandemic disease. By March 16, 2020, Canada closed its borders and adopted a model of "social distancing" as a preventative measure to slow down community transmission. COVID-19 Status (17-Mar-2020): Cases: 198,348; Deaths 7,979, Recovered: 82,763.
Current Consensus on Emerging Coronavirus Species and Related Diseases
The Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, the international entity responsible for the classification of viruses and taxon nomenclature of the family Coronaviridae, recently reclassified the original strain identified as 2019-nCoV[2] naming it SARS-CoV-2[10].
The rationale for the change rests on the fact that the original virus strain identified as 2019-nCoV[2] represents a sister common ancestor to the prototype human and bat severe acute respiratory syndrome coronaviruses (SARS-CoVs) of the species Severe acute respiratory syndrome-related coronavirus[10].
This denotation and comparison not only explains the high level of sequence homology (96.2%) shared between the human SARS-CoV-2 and the bat SARSr-CoV RaTG13 strain[2,10] but also the zoonotic or potential direct transmission of SARS-CoV-2 from bats to humans without the need of an intermediate animal reservoir. Contrary to two related coronavirus infections, Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS), affecting humans, no clear intermediate reservoir of SARS-CoV-2 has been reported.
The high homology between human SARS-CoV-2 and bat SARSr-CoV RaTG13 (96.2%) coronavirus together with the reported selling of wild animals at the Huanan Seafood Wholesale Market, considered to be the epicentre of the first COVID-19 outbreak, calls for a closer look at current practices in Asia, Africa, Oceanic Islands, Central America and South America concerning bat hunting for ingestion as meat and medicine.
Apart from direct exposure of humans to a variety of infectious diseases including viruses from the Coronaviridae family, this practice has significantly affected 167 species of bats around the world severely threatening their survival[11].
In the case of the 2002/2003 SARS outbreak, it is believed that the coronavirus, SARS-CoV, the causative agent of SARS, might have depended upon virus transmission between bats and civet cats prior to its transmission into humans[12]. In the case of MERS-CoV, the coronavirus responsible for the MERS outbreak in 2012, it appears that the intermediate reservoir of this virus was dromedary camels[13].
Although early reports suggested that pangolins or scaly anteaters might be an intermediate reservoir of SARS-CoV-2 transmission to humans[14], an in-depth sequence analysis of virome data sets from pangolins and SARS-related coronaviruses from bats, particularly around the spike glycoprotein, suggested that human SARS-CoV-2 did not come directly from pangolins[15].
The significantly higher level of homology between human SARS-CoV-2 and the bat SARSr-CoV RaTG13 strain (96.2%)[2], by comparison to those found between SARS-CoV-2 and SARS-CoV (79.6%)[2,6] and between SARS-CoV-2 and MERS-CoV(50%)[6], respectively, is consistent with a potentially more direct transmission from bats, being the original hosts, to humans.
Since the original new strain isolates and their sequence analysis leading to the discovery of SARS-CoV-2 were obtained from the first cluster of seven patients treated at the intensive care unit (ICU) of the Wuhan Jin Yin-Tan Hospital at the beginning of the outbreak, between December 20 and December 29, these patients were most likely all exposed to the same potential intermediate animal reservoir.
Indeed, with the exception of one patient, six patients reported to be wholesalers or delivery workers at the Huanan Seafood Wholesale Market[2] located in the city of Wuhan, the capital of Hubei province, within central China.
As mentioned in the first section, SARS-CoV-2/COVID-19 Outbreak, it was reported by local Chinese authorities that, apart from seafood, the Huanan Seafood Wholesale Market also sold wild animals. It is highly possible that the intermediate animal reservoir of SARS-CoV-2 could be found within one of such wild animals available at the market during the days preceding the COVID-19 outbreak.
Based upon these findings, it would be of interest to the international community to encourage the Chinese Legislative Affairs Commission and corresponding top authorities to continue the current ban of selling wild animals beyond the current COVID-19 outbreak and champion the global ban of trading wild animals in all other countries with similar practices.
This is of particular relevance for immediate early prevention of future cyclic outbreaks of Coronaviridae affecting humans. This is due to the fact that, once potentially infective viral particles are transmitted from intermediate reservoirs to humans, human to human transmission rates seem to be remarkable[6,15].
Current Consensus on the official naming of species of the Coronaviridae family
causing Severe Acute Respiratory Syndrome (SARS)/Acute Respiratory Distress Syndrome (ARDS)
In perhaps one of the most in-depth and elegant reports of the Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV), the need to promptly articulate and disseminate the phylogeny and universal use of a consensus taxonomic classification of emerging coronavirus affecting human health was recently published[10].
Some of the important highlights published by the CSG are as follows:
- The original viral strains isolated and sequenced from the first cluster of patients within Wuhan, capital of Hubei, central China, originally known as 2019-nCoV[2], is a "sister group" or clade to human and bat Severe Acute Respiratory Syndrome Coronaviruses (SARS-CoVs).
- It is proposed that the new coronavirus originally known as 2019-nCoV[2] be re-named and identified as SARS-CoV-2.
- Future individual strains derived from SARS-CoV-2 should be named as follows:
SARSCoV-2/host/location/isolate/date.
Figure 2 illustrates additional practical information published by CSG[10]. It is highly desirable that this information be readily shared with the public at large in order to further clarify the exact name of the diseases that different Severe Acute Respiratory Syndrome Coronaviruses (SARS-CoVs) have caused in recent years, how they relate to each other and their impact on human health, globally.
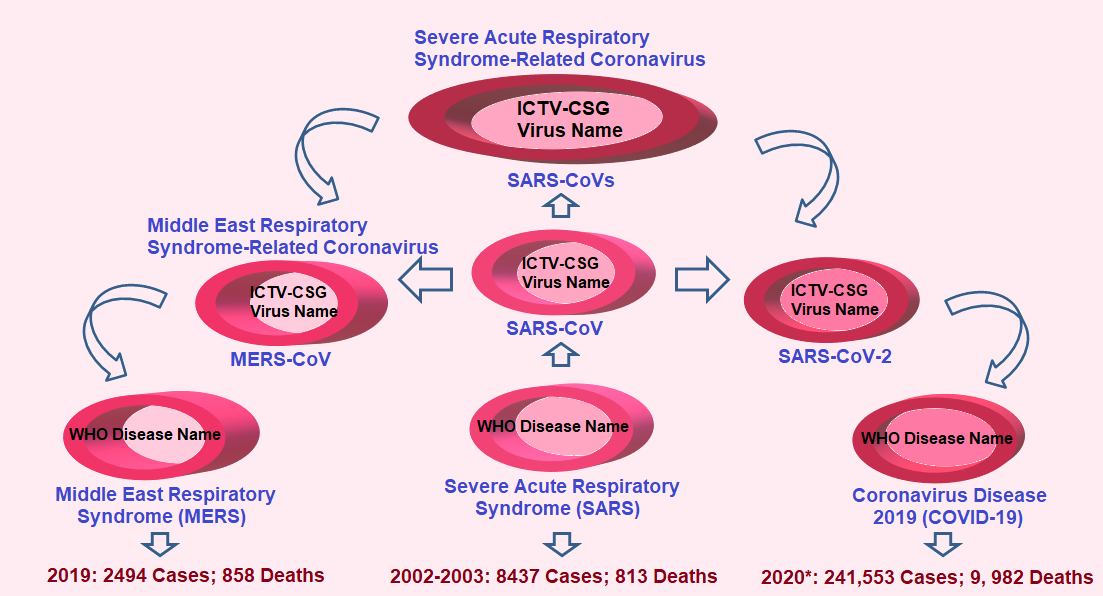
Figure 2 Consensus on the name of Severe Acute Respiratory Syndrome-Related Coronavirus (SARS-CoVs) and on the name of the diseases caused by these viruses
Consensus on the name of new strains of coronavirus related to recent outbreaks, including COVID-19, were recently published by the Coronaviridae Study Group (CSG) of the International Committee on Taxonomy of Viruses (ICTV)[10]. The corresponding names of the diseases caused by Severe Acute Respiratory Syndrome-Related Coronavirus (SARS-CoVs), as established by the World Health Organization (WHO), are also depicted. The number of cases and deaths by each of the most recent outbreaks are also provided for comparison. WHO data for SARS and MERS are in clear contrast with current estimations of COVID-19 pandemic outbreak.
*Number of cases and deaths for COVID-19 represent estimates as of March 19, 2020.
Table 2 summarizes the current consensus in relation to Severe Acute Respiratory Syndrome (SARS)/Acute Respiratory Distress Syndrome (ARDS) caused by viruses from the Coronaviridae family in accordance with the World Health Organization (WHO) and the International Committee on Taxonomy of Viruses (ICTV)[10], including total number of cases, cumulative number of deaths and case fatality rates for each disease.
|
CSG/ICTV Virus |
WHO Disease |
Cases |
Cumulative Numbers Deaths |
Case Fatality Rate % |
| SARS-CoV | SARS | 8437 | 813 | 9.6 |
| MERS-CoV | MERS | 2494 | 858 | 34.4 |
| SARS-CoV-2 | COVID-19 | 338,724 | 14,687 | 4.34 |
Table 2 Summary of cases, deaths and fatality rates caused by Severe Acute Respiratory Syndrome-Related Coronavirus (SARS-CoVs)
Since the first global outbreak of Severe Acute Respiratory Syndrome (SARS) in 2002/2003, Severe Acute Respiratory Syndrome-Related Coronavirus (SARS-CoVs) have been responsible for two additional and rather contemporary outbreaks, MERS and COVID-19. Although MERS appears to continue representing the SARS-CoVs-related disease with the highest case fatality rate (34%), COVID-19 has led to more deaths (14,687) than SARS and MERS combined (1,671). WHO data for SARS and MERS; COVID-19 data represents current available estimates as of March 23, 2020.
SARS-CoV-2 viral entry: Endocytosis through non-clathrin
non-caveolae viral Spike protein/ACE2 host receptor-mediated processing
As shown in Figure 3A, SARS-CoV-2 binds to host cells through binding of its Spike protein to Angiotensin Converting Enzyme 2 (ACE2) in a similar fashion as SARS-CoV[2,9,16].
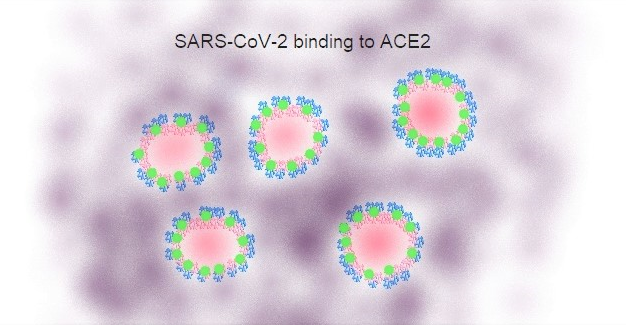
Figure 3A Selective binding of SARS-CoV-2 to Host Cells
Binding of SARS-CoV-2 (in blue) to host cells, primarily from the lower respiratory tract, takes place by interaction of the virus receptor-binding spike protein encoded by the S gene with its putative receptor Angiotensin Converting Enzyme 2 (ACE2, in green)[2,9]. Binding to ACE2 seems to be a common entry path between SARS-CoV and SARS-CoV-2 but not MERS-CoV.
ACE2 seems to be a common cell-surface receptor for both SARS-CoV and SARS-CoV-2. Meanwhile, MERS-CoV cell-surface binding is most likely mediated through its interaction with CD26 (dipeptidyl peptidase 4 [DPP4]) as its putative receptor[17]. The reported binding of SARS-CoV-2 to ACE2[2,9] leads to the possibility of similar viral entry mechanisms shared between SARS-CoV and SARS-CoV-2.
Briefly, as appears to be the case with SARS-CoV, we have advanced the concept that SARS-CoV-2 may also enter targeted lower respiratory cells through a non-clathrin, non-caveolae ACE2-mediated endocytosis. It is highly likely that, following viral anchoring to host cell membrane and early processing through a pH-dependent mechanism similar to that found in Transferrin-Transferrin Receptor complexes, early and late endosome mediated entry of SARS-CoV-2 takes place[18-20].
Figure 3B illustrates the proposed binding and endosome-mediated entry of SARS-CoV-2/ACE2 viral receptor complexes into low respiratory host cells as well as a possible intracellular mechanism for the release of infectious viral particles.
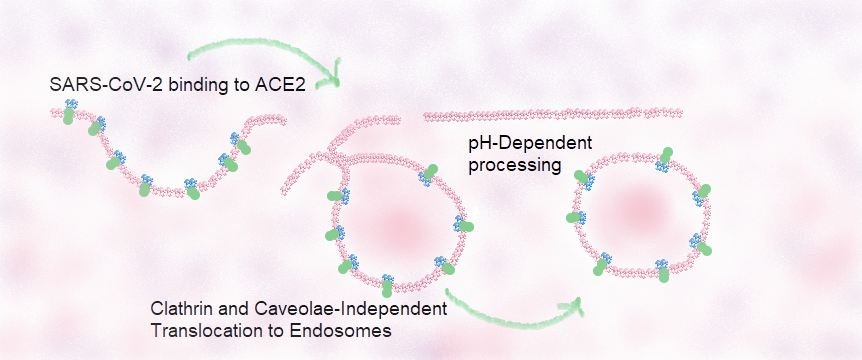
Figure 3B Proposed SARS-CoV-2 endosome-mediated entry of SARS-CoV-2/ACE2 viral receptor complexes into lower respiratory host cells
SARS-CoV-2 (in blue) selective cell-surface binding takes place through its interaction with Angiotensin Converting Enzyme 2 (ACE2 in green), known to be the putative receptor of SARS-CoV-2[9]. As in the case of SARS-CoV, processing of this complex is most likely mediated by the early and strong binding of the receptor binding domain (RBD) of SARS-CoV-2 spike S1 protein domain to the Angiotensin Converting Enzyme 2 (ACE2) receptor, followed by early entry through endosome formation after fusion between viral and host cell membranes mediated by the interaction of SARS-CoV-2 spike S2 protein domain within the RBD and ACE2 complexes[21]. Late entry of SARS-CoV-2 might be dependent upon a pH-dependent processing by means of the activation of an ATP-dependent proton translocating pump of late endosomes containing SARS-CoV-2/ACE2 complexes[20].
Based upon the combined similarities between the binding of SARS-CoV and SARS-CoV-2 to ACE2 receptor of host cells, together with a late endosomal pH-dependent processing of S1/S2 Spike binding domains within SARS-CoV-2/ACE2 receptor complexes, on February 27, 2020, we advanced the concept of the use of lysosomotropic agents to hinder the infectivity of SARS-CoV-2 as an effective prophylactic tool to abrogate community transmission of COVID-19.
In addition, through the use of gene-editing technologies[22] to attenuate Spike protein binding capacity and infectivity of SARS-CoV-2, as well as the adjuvant use of an array of some of our nNE2-nNE7 biogenic clones, together with some currently approved lysosomotropic agents, we are currently pursuing their industrial manufacturing and widespread use to mitigate community infectivity particularly within the high risk populations.
It is hoped that this approach will enable faster, more robust and earlier preventative interventions to reduce current high transmission rates as well as attenuating the higher number of deaths caused by COVID-19 (14,687) in comparison to those incurred by SARS and MERS, combined (1,671).
From a broader and global preventative approach to the onset of inevitable upcoming emerging outbreaks related to Severe Acute Respiratory Syndrome Related Coronavirus (SARS-CoVs), the following International Strategies are highly desirable:
- Introduce a systematic and validated international sentinel programme for the analysis of genome composition and divergence of nCoVs in known and potential animal reservoirs.
- Based upon the critical role and relatively conserved sequences of SARS-CoVs Spike S viral peptides, commence the articulation of immune-based preventative therapeutics and reverse-vaccine modelling and omics next-generation vaccine programmes for Global Public Health.
- Support an International effort to promote a permanent ban on the trading of all wild animals for Bushmeat and Medicine, in particular that of Bats in Asia, Africa, Oceania, Central America and South America, and a ban of trophy hunting which equally leads to local consumption of the meat of carcases left behind.
In closing, our Centre remains convinced and extremely encouraged by the arsenal of exquisite and powerful biological technologies that can be brought to bear on this pandemic with ethical and speedy timelines for the betterment of humanity and of all living species to reduce even the most historical and adverse infectious diseases such as COVID-19 and many more that will arise in the immediate future. We continue to be committed to supporting vital Global Preventative Health Initiatives in a decisive and collaborative fashion.
For reprints, please use the contact form, below, or address the corresponding author, listed above.
