Journal of Health Sciences Observational News (JHSON)™
Gene-editing: Translating bacterial adaptive immunity into promising cancer therapies
Dr Gabriel Pulido-Cejudo1,2*, Peter Humphries2 and Khadija El Abdaimi1,2
1International Centre for Advancement of Health Regional Innovation and Science, ICAHRIS
*corresponding author, gabriel.pulido-cejudo@icahris.org
2Canadian Federation of Breast Diseases, CFBD
Article
Close to five decades ago, our understanding about how bacteria can overcome adverse events, such as exposure to chemicals, detergents, antibiotics and DNA-containing viruses, granted us the ability to use some of the molecules and enzymes involved in bacterial DNA protection and repair to perform DNA editing and cloning of human and other genes of interest[1-4].
To date, the advancements within the field of gene editing have achieved ever greater levels of sophistication and refinement, making possible the development of less invasive and more precise cancer therapies, amongst other diverse applications. Three levels of refinement relate to the use of a prokaryotic or bacterial adaptive immune system that takes advantage of key elements deployed by bacteria as a mechanism of defence against foreign viral DNA.
The first level of refinement refers to the identification of discrete, repeated DNA sequences, or palindromes, that are identical when they are read from left to right (5' end to 3' end in blue, top sequence) and from right to left (3' end to 5' end in green, bottom sequence) as shown below:
5' TGGCCA 3'
3' ACCGGT 5'
As DNA sequences are transcribed into messenger RNA (mRNA) molecules, the resulting mRNA sequences are complementary to the corresponding DNA being transcribed. This process, in essence, enables one to identify selective sequences of DNA based upon the analysis of complementary mRNA molecules.
Through years of research focused on deciphering the biological significance and evolutionary traits of various types of DNA and RNA molecules found in bacterial plasmids and other prokaryotes, the second level of refinement towards selective gene editing encompasses the identification and characterization of clustered palindromic sequences separated by non-palindromic sequences. These clustered palindromic sequences were identified with the putative name of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)[5,6].
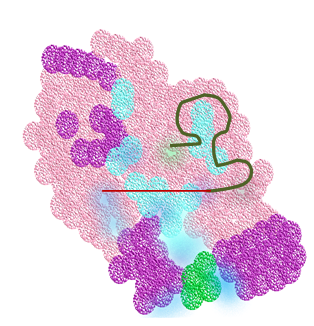
The third and last level of refinement concerning the most current and powerful level of gene editing relates to the association of bacterial DNA endonucleases, capable of selectively cutting discrete DNA sequences with CRISPR. In particular, the Cas9 endonuclease leads to the formation of the CRISPR-Cas9 system (Fig.1). Briefly, the CRISPR-Cas9 system consists of two major components.
The first involves a Cas9 endonuclease, and the second component is a specific type of RNA called single-stranded guide RNA (sgRNA). When viral DNA infects bacteria, the CRISPR-Cas9 system, guided by sgRNA, associates with a CRISPR RNA (crRNA) and a trans-activating crRNA (tracrRNA). The Cas9 endonuclease proceeds to bind to a short DNA motif, a Protospacer Adjacent Motif (PAM), cleaving the exogenous viral DNA complementary to the crRNA guide with a high level of fidelity and precision[7]. Once the undesirable exogenous viral invading sequences are selectively removed, the affected DNA is repaired. This extraordinary CRISPR-Cas9 system is now being used to perform highly selective gene editing in eukaryotic cells including human cancerous and stem cells.
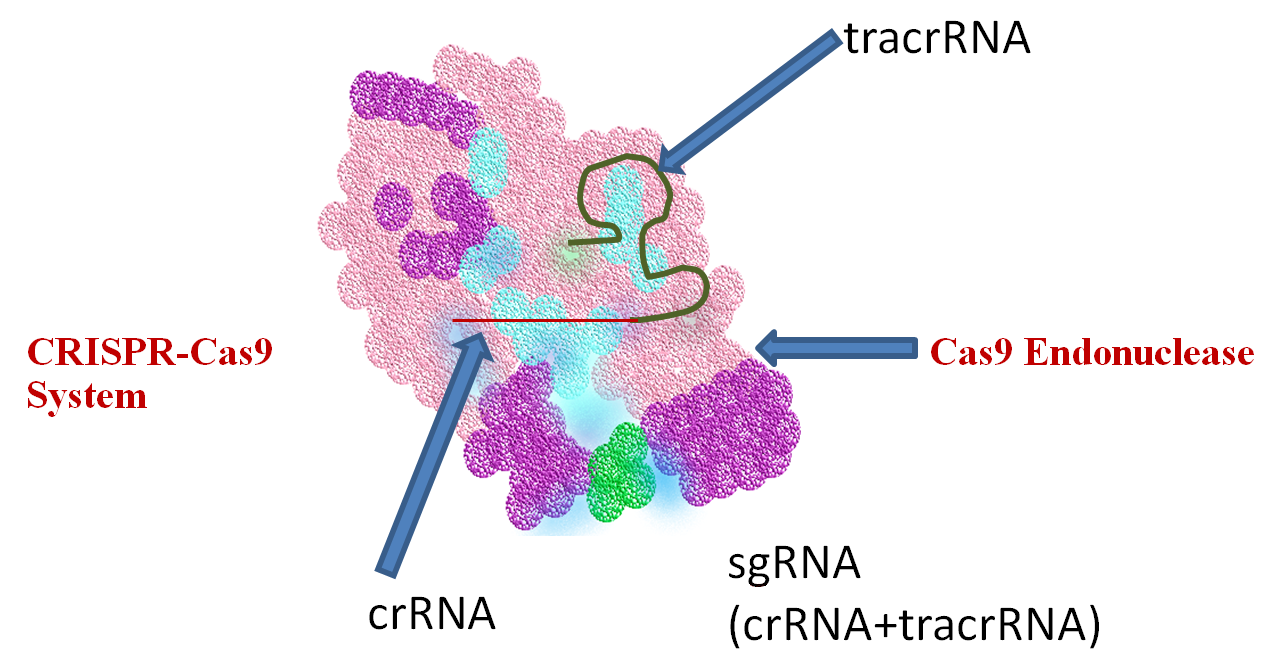
Figure 1 CRISPR-Cas9 System and its function in gene editing. Through adaptive evolution of bacterial immunity to infectious viral particles (bacteriophage), bacteria developed a refined gene editing system, known as CRISPR-Cas9, capable of identifying and removing undesirable viral DNA sequences, followed by bacterial DNA repair. This same system has led to the most powerful and promising tools to perform refined gene editing in the DNA of human cells, harnessing one of the most elegant and least invasive approaches to correct genetic defects and chronic ailments such as cancer.
Current advances in the development of CRISPR-Cas9-based functional gene therapies
Apart from conferring bacteria an elegant, simple yet precise mechanism to protect themselves against, amongst others, foreign DNA viral sequences, the CRISPR-Cas9 system / loci has been brought to a new level of selective DNA editing in cells other than bacteria. Indeed, during the last seven years, the CRISPR-Cas9 system has proven to be a viable conduit by which to tailor refined, specific therapies against various cancer types affecting humans[8]. As revealed in Table 1, the main cancer applications developed have targeted primarily leukaemia, lung, breast, liver and ovarian cancer.
| Cancer Type |
Applications in % relative to all other cancer types |
| Leukaemia | 16.4 |
| Lung | 14.3 |
| Breast | 11.7 |
| Liver | 9.8 |
| Ovarian | 9.4 |
| Renal Cancer | 8.9 |
| Colorectal | 7.9 |
| Lymphoma | 6.6 |
| Melanoma | 4.9 |
| Glioma | 3.6 |
| Cervical | 2.1 |
| Gastric | 2.1 |
| Esophageal | 1.3 |
| Thyroid | 1.1 |
Table 1 CRISPR-Cas9 Applications in Cancer. During the last seven years, efforts to develop less invasive therapies against cancer using the CRISPR-Cas9 system have encompassed various approaches, including functional gene therapy and drug discovery primarily targeting leukaemia, lung, breast, liver and ovarian cancers.
Current clinical trials using the CRISPR-Cas9 system targeting various types of cancers are based upon the use of monoclonal antibodies, a type of highly selective engineered antibodies that can bind to specific cellular molecules of interest and inhibit their function. In particular, because monoclonal antibodies against the programmed cell death protein-1 (PD-1) have proven to be successful in the treatment of lung and other cancer types, this specific T-cell receptor has been selected as a target for CRISPR-Cas9 gene editing. Under normal circumstances, the programmed cell death protein-1 (PD-1) T-cell receptor inhibits T-cell activation, facilitating immune tolerance and preventing autoimmune reactions [8,9]. However, during the emergence of cancerous cells, PD-1 activation and, therefore, attenuation of immune response enables these cells to avoid destruction by T-cell activation and their selective killing of cancerous cells.
Instead of blocking the continuous production of PD-1 with selective monoclonal antibodies, the CRISPR-Cas9 gene editing system enables the deletion of the specific gene responsible for the production of PD-1. This process provides the ability to create T-cells that can activate the immune response without attenuation by programmed cell death protein-1 (PD-1). As shown in Figure 2, the first step to treat cancer patients with the CRISPR-Cas9 system consists of taking a blood sample. Using conventional methods, T cells from cancer patients are isolated and purified. Once purified, the selective region encoding the PD-1 protein in the isolated T-cells is deleted through CRISPR-Cas9 selective gene editing. Gene-edited T-cells lacking PD-1 are further purified and grown outside the patient's body through ex-vivo expansion of these cells. Once a desirable number of CRISPR-Cas9 gene-edited T-cells lacking PD-1 is obtained, these cells are injected back into the cancer patient. CRISPR-Cas9 gene-edited T-cells lacking PD-1 injected into cancer patients are now able to induce a T-cell response against cancerous cells.
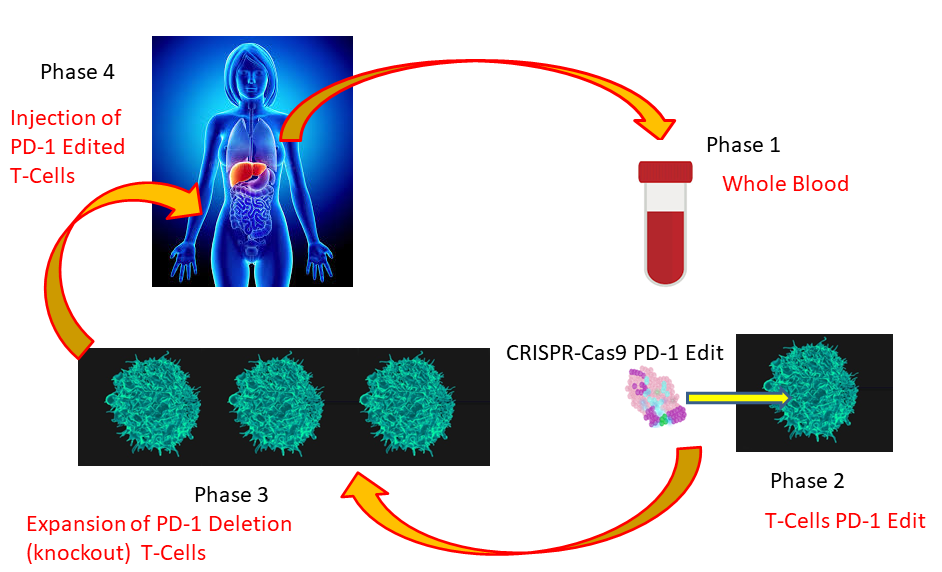
Figure 2 CRISPR-Cas9 PD-1 Editing of T-Cells from Cancer Patients. Current clinical trials using CRISPR-Cas9 have primarily concentrated on the elimination (knockout) by gene editing of the programmed cell death protein-1 (PD-1) responsible for attenuating the T-cell immune response. Phase 1 consists of obtaining whole blood from a cancer patient. Phase 2 involves the purification of T-cells and gene editing of the PD-1 protein. During Phase 3, edited T-cells are purified and expanded through ex-vivo cell cultures. In Phase 4, expanded, edited T-cells lacking PD-1 are transfused back into the cancer patient. Since gene-edited T-cells lack PD-1 mediated immune attenuation, these edited T-cells are expected to promote a T-cell mediated immune response against cancerous cells.
The onset of cancer has proven to be, in most cases, the result of a complex and multifactorial set of events altering not only how affected cells reproduce but also how they interact with surrounding tissues, blood vessels, our immune network and supportive tissues through a concerted cascade of metabolic signals. As cancer progresses, the complexity of tumour growth and the invasion of other tissues and organs increases the number of factors, molecular and cellular events, that are affected. From DNA replication and repair to DNA transcription, translation and crosstalk amongst cells, the progression of oncogenic signalling pathways and the metastasis or invasion of other tissues becomes more complex and ill-understood. It is for this reason that, in addition to current conventional pharmaceuticals and monoclonal antibody-based selective immunotherapies, the genomic landscape of cancerous cells has seen the addition of another level of anti-cancer agents to its repertoire. Indeed, during the last two years, the number of clinical trials involving promising CRISPR-Cas9 system gene-editing therapies has increased. Table 2 outlines current clinical trials tailored against different types of cancer using CRISPR-Cas9 system gene-editing therapies.
| Cancer Type(s) | Target | Trial Phase | Gene Editing Strategy | Clinical Trial Identifier |
| Advanced Esophageal | PD-1 | II | PD-1 knockout T-cells | NCT03081715 |
| Bladder | PD-1 | I | PD-1 knockout T-cells | NCT02863913 |
| B cell lymphoma / leukaemia | CD19 | I / II |
CAR-T cells CD19 edit |
NCT03166878 |
| B cell lymphoma / leukaemia | ___ | ___ |
CAR-T cells CD19 and CD20 or CD22 |
NCT03398967 |
| Castration resistant prostate | PD-1 | I | PD-1 knockout T-cells | NCT02867345 |
| CD19+ leukaemia / lymphoma | TCR | I | TCR and B2M | NCT03166878 |
| EBV associated malignancies | PD-1 | I / II | PD-1 knockout T-cells | NCT03044743 |
| HPV-related malignant neoplasm |
HPV16-E6/E7 HPV18-E6/E7 |
I | HPV16-E6/E7 or HPV18-E6/E7 knockout | NCT03057912 |
| Metastatic non-small cell lung carcinoma | PD-1 | I | PD-1 knockout T-cells | NCT02793856 |
| Metastatic renal cell carcinoma | PD-1 | I | PD-1 knockout T-cells | NCT02867332 |
| Muscle-invasive bladder | PD-1 | I | PD-1 knockout T-cells | NCT02863913 |
|
Multiple myeloma / melanoma Synovial sarcoma Myxoid / round cell liposarcoma |
TCR (myeloma) PD-1 (melanoma) |
I | TCR and PD-1 knockout T-cells | NCT03399448 |
| Non small-cell lung carcinoma | PD-1 | I | PD-1 knockout T-cells | NCT02793856 |
| Relapsed / refractory leukaemia and lymphoma | CD19 and CD20 or CD22 | I / II | CD19 and CD20 or CD22 | NCT03398967 |
| Renal cell carcinoma | PD-1 | I | PD-1 knockout T-cells | NCT02867332 |
| Tumour Central Nervous System | NF1 | ___ | Fix NF1 mutation allele | NCT03332030 |
Table 2 Current Clinical Trials using CRISPR-Cas9 mediated gene-editing for cancer. Data was retrieved on 20-Aug-2019 from the repertoire deployed in clinicaltrials.gov. B2M, Beta-2-Microglobulin CD (19, 20 and 22) Cluster of Differentiation 19, 20 and 22; CAR-T Cells, Chimeric Antigen Receptor-T cells; HPV, 16 and 18, Human Papilloma Virus 16 and 18; NF-1 Neurofribromin 1; TCR, T-cell receptor.
In addition to the continuous emergence of numerous applications of the CRISPR-Cas9 System for the analysis, diagnosis and treatment of chronic diseases such as cancer as well as of infectious and emerging diseases, CRISPR-Cas9 gene-editing technology has equally unlocked a powerful yet simple pathway to decrease the vulnerability of endangered animals, plants and all other living species, globally. With so many empowering and promising applications for the betterment of humanity and of all life on earth, it is our stern desire and commitment to deter the foreseen misuse of CRISPR-Cas9 for unethical purposes including the weaponization of life-threatening microorganisms.
In the course of the next decade, CRISPR-Cas9 System gene editing technology will undoubtedly represent a central enabling technology for life extension and reversal of aging, increasing the quality of life for an ever-aging population in the most remarkable way.
For reprints, please use the contact form, below, or address the corresponding author, listed above.